Jagran Josh
CBSE Class 10 Chemistry Chapter 1 Important Questions: Central Board of Secondary Education is likely to release the 2023 class 10th board examination date sheet soon. The board has not yet announced a fixed date for the release of the date sheet.
The board exams are likely to commence in February, 2023.
With about two months left for CBSE Class 10 Science board examinations, students should begin their preparations if they haven’t already done so.
In this article, we are going to discuss the Important questions of CBSE Class 10 Chemistry Chapter 1 Chemical Reactions and Equations. It is the first chapter in Unit I Chemical Substances – Nature and Behaviour.
CBSE Class 10 Chemistry Chapter 1 Important Questions:
Unit I: Chemical Substances – Nature and Behaviour
Chapter 1 Chemical Reactions and Equations
MULTIPLE CHOICE QUESTIONS
- When Ag is exposed to air it gets a black coating of
(a) AgNO3
(b) Ag2S
(c) Ag2O
(d) Ag2CO3
- Which of the reactions is used in black and white photography?
(a) Combination Reaction
(b) Decomposition Reaction
(c) Displacement reaction
(d) Oxidation reaction
MnO2 + 4HCl →MnCl2 + 2H2O + Cl2
- Identify the substance oxidized in the above equation.
(a) MnCl2
(b) HCl
(c) H2O
(d) MnO2
- Zinc reacts with silver nitrate to form which compounds?
(a) Zn (NO3)2 + Ag (b) ZnNO3 + Ag (c) AgNO3 + Zn (NO3)2 (d) Ag + Zn (NO3)3
- In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be used in place of lead nitrate?
- a) Lead sulphate (insoluble)
(b) Lead acetate
(c) Ammonium nitrate
(d) Potassium sulphate
- The brown gas evolved on heating of copper nitrate is
(a) O2
(b) NO2
(c) N2
(d) NO
- Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is:
(a) 1: 1
(b) 2:1
(c) 4:1
(d) 1:2
- A substance ‘X’ is used in white-washing and is obtained by heating limestone in the absence of air. Identify ‘X’.
(a) CaOCl2
(b) Ca (OH)2
(c) CaO
(d) CaCO3
- 2HNO3 + Ca (OH)2 → Ca (NO3)2 + 2H2O; is an example of
(i) displacement reaction
(ii) double displacement reaction
(iii) neutralisation reaction
(iv) combination reaction.
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iv)
- A substance X which is a group 2 element is used intensively in the cement industry. This element is present in bones also. On treatment with water, it forms a solution which turns red litmus blue. Element X is
(a) Cu
(b) Ca
(c)Na
(d) Al
ASSERTION- REASON TYPE QUESTIONS
DIRECTION: Each of these questions contains an assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
(a) If both Assertion and Reason are correct and reason is the correct explanation of Assertion.
(b) If both Assertion and Reason are correct, but reason is not the correct explanation of Assertion.
(c) If Assertion is correct but Reason is incorrect.
(d) If Assertion is incorrect but Reason is correct.
Q1. Assertion (A): Photosynthesis is considered as an endothermic reaction.
Reason (R): Energy gets released in the process of photosynthesis
Q2. Assertion (A): MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O is redox reaction.
Reason (R): MnO2 oxidises HCl to Cl2 and gets reduced to MnCl2.
Q3. Assertion (A): When HCl is added to zinc granules, a chemical reaction occurs.
Reason (R): Evolution of a gas indicates that the chemical reaction is taking place.
Q4. Assertion (A): White silver chloride turns grey in sunlight.
Reason (R): Decomposition of silver chloride in presence of sunlight takes place to form silver metal and chlorine gas.
Q5. Assertion (A): Chemical reaction changes the physical and chemical properties of a substance
Reason (R): Chemical change involves a change in the chemical composition of matter, and a new substance is formed
CASE STUDY QUESTIONS
- A chemical reaction is a representation of chemical change in terms of symbols and formulae of reactants and products. There are various types of chemical reactions like combination, decomposition, displacement, double displacement, oxidation and reduction reactions. Reactions in which heat is released along with the formation of products are called exothermic chemical reactions. All combustion reactions are exothermic reactions.
(i) The massive force that pushes the rocket forward through space is generated due to the
(a) combination reaction
(b) decomposition reaction
(c) displacement reaction
(d) double displacement reaction
(ii) A white salt on heating decomposes to give brown fumes and yellow residue is left behind. The yellow residue left is of
(a) lead nitrate
(b) nitrogen oxide
(c) lead oxide
(d) oxygen gas
(iii) Which of the following reactions represents a combination reaction?
(a) CaO (s) + H2O (l) → Ca (OH)2 (aq)
(b) CaCO3 (s) → CaO (s) + CO2(g)
(c) Zn(s) + CuSO4 (aq) → ZnSO4 (aq) + Cu(s)
(d) 2FeSO4(s) → Fe2O3 (s) +SO2(g) + SO3(g)
(iv) Complete the following statements by choosing correct type of reaction for X and Y.
Statement 1: The heating of lead nitrate is an example of ‘X’ reaction.
Statement 2: The burning of magnesium is an example of ‘Y’ reaction.
(a)X-Combination,Y-Decomposition
(b)X-Decomposition,Y-Combination
(c)X-Combination,Y-Displacement
(d) X- Displacement, Y-Decomposition
- Those reactions in which two compounds react by an exchange of ions to form two new compounds are called double displacement reactions. A double displacement reaction usually occurs in solution and one of the products, being insoluble, precipitate out (separates as a solid). Any reaction in which an insoluble solid (called precipitate) is formed that separates from the solution is called a precipitation reaction. The reaction in which acid or acidic oxide reacts with base or basic oxide to form salt and water is called neutralisation reaction.
For example, 2NaOH+H2SO4⟶Na2SO4 + 2 H2O
(i) When hydrogen sulphide gas is passed through a blue solution of copper sulphate, a black precipitate of copper sulphide is obtained, and the sulphuric acid so formed remains in the solution. The reaction is an example of a
(a) combination reaction
(b) displacement reaction
(c) decomposition reaction
(d) double displacement reaction
(ii) Barium chloride on reaction with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved?
(I) Displacement reaction
(II) Precipitation reaction
(III) Combination reaction
(IV) Double displacement reaction
(a) (I) only
(b) (II) only
(c) (III) and (IV) only
(d) (II) and ( V) only
(iii) Identify A in the following reaction.
AlCl3(aq) + 3NH4OH (aq)⟶A + 3NH4Cl(aq)
(a) Al (OH)3
(b) Al2 O3
(c) AlH3
(d) AlN
(iv) Consider the following reaction, BaCl2 + Na2SO4⟶ BaSO4 + 2NaCl. Identify the precipitate in the reaction,
(a) BaCl2
(b) BaSO4
(c) Na2SO4
(d) NaCl
Short Answer Type Questions-I (2 Marks)
- You might have noted that when copper powder is heated in a China dish, the reddish brown surface of copper powder becomes coated with a black substance.
(AI 2019)
(a) Why is this black substance formed?
(b) What is the black substance?
(c) Write the chemical equation of the reaction that takes place.
(d) How can the black coating on the surface be turned reddish brown?
- Define a combination reaction. Give one example of a combination reaction which is also exothermic.
(a) A solution of potassium chloride when mixed with silver nitrate solution, an insoluble white substance is formed. Write the chemical reaction involved and also mention the type of the chemical reaction.
(b) Ferrous sulphate when heated, decomposes with the evolution of a gas having a characteristic odour of burning sulphur. Write the chemical reaction involved and identify the type of reaction.
(Board Term I, 2016)
- What is observed when carbon dioxide gas is passed through lime water.
(i) For a short duration
(ii) For long duration?
Also, write the chemical equations for the reaction involved.
[Board Term I, 2016]
- Lead nitrate solution is added to a test tube containing potassium iodide solution.
(a) Write the name and colour of the compound precipitated.
(b) Write the balanced chemical equation for the reaction involved.
(c) Name the type of this reaction justifying your answer.
(2020)
- Name the type of chemical reaction represented by the following equation:
(Board Term I, 2016)
(i) CaO + H2O → Ca(OH)2
(ii) 3BaCl2 + Al2(SO4)3 → 2AlCl3 + 3BaSO4
(iii) FeSO4→HeatFe2O3+SO2+SO3
- 2 g of silver chloride is taken in a China dish and the China dish is placed in sunlight for some time. What will be your observation in this case? Write the chemical reaction involved in the form of a balanced chemical equation. Identify the type of chemical reaction.
(Delhi 2019)
- List four observations that help us to determine whether a chemical reaction has taken place.
[Board Term-I, 2012]
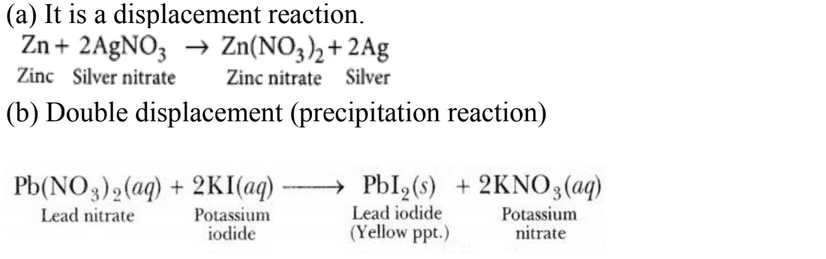
- Identify the type of reactions taking place in each of the following cases and write the balanced chemical equation for the reactions.
(a) Zinc reacts with silver nitrate to produce zinc nitrate and silver.
(b) Potassium iodide reacts with lead nitrate to produce potassium nitrate and lead iodide.
(Delhi 2019)
- i) Can a displacement reaction be a redox reaction? Explain with the help of an
example.?
- ii) Identify the substances that are oxidised and the substances that are reduced in the
following reactions.
(Board Term I, 2015)
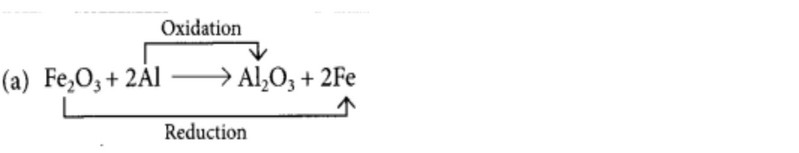
(a) Fe2O3 + 2Al → Al2O3 + 2Fe
(b) 2PbO + C → 2Pb + CO2
Also check: CBSE Class 10 Science Syllabus 2022-23
Answers to CBSE Class 10 Chemistry Chapter 1 Important Questions:
ANSWERS:
MULTIPLE CHOICE QUESTIONS
- b
- b
- b
- a
- b
- b
- b
- c
- b
- b
ASSERTION- REASON TYPE QUESTIONS
- c
- a
- a
- a
- a
CASE STUDY QUESTIONS
- i) (b)
The massive force that pushes the rocket forward through space is generated due to the decomposition reaction. Hydrogen peroxide decomposes and provides it with a considerable reaction force thrust.
- ii) (c)
Lead nitrate decomposes to give brown fumes of nitrogen dioxide gas and yellow residue of lead oxide is left behind.
iii) (a)
A reaction in which two or more reactants combine to form a single product is known as a combination reaction.
- i) (d)
- ii) (d)
iii) (a)
- iv) (b)
SHORT ANSWER QUESTIONS
2. A combination reaction is said to have occurred when two or more than two substances combine to form a single substance.
CaO + H2O → Ca(OH)2
3.
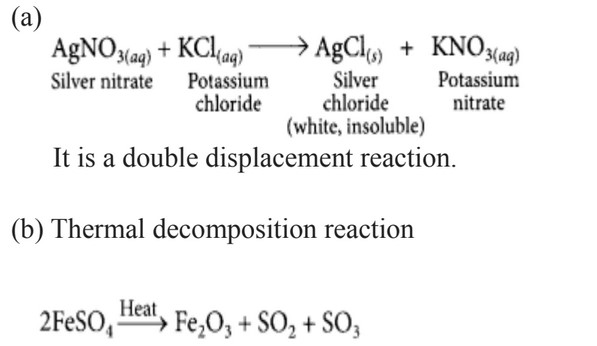
4. (i) For short duration: Limewater turns milky due to the formation of CaCO3, Which is insoluble in water. CaOH2 + Co2 → CaCo3 + H2O
(ii) For Long duration: A clear solution is obtained due to the formation of calcium bicarbonate. Ca (HCO3)2 which is soluble in water. CaCO3 + H2O → Ca(HCO3)2
5. (a) When lead nitrate is added to potassium iodide then yellow precipitate of lead iodide is formed along with potassium nitrate.
(b) Balanced chemical reaction is as follows : Pb(NO3)2 + 2KI → PbI2 + 2KNO3
6. (i) Combination reaction.
(ii) Precipitation reaction or double displacement reaction.
(iii) Thermal decomposition reaction.
7. When 2 g of silver chloride is taken in a china dish and the china dish is placed in sunlight for some time, the white sodium chloride turns grey due to the decomposition reaction through the sunlight that decomposes silver chloride into silver and chlorine by light. 2 AgCl (s) → 2 Ag (s) + Cl2 (g)
8. (i) Evolution of gas
(ii) Change in temperature
(iii) Change in state
(iv) Change in colour
9.
10.
(i) Consider the following displacement reaction: Zn(s)+ CuSO4(aq) → ZnSO4(aq) + Cu(s) Here, Zn has changed into ZnSO4 (i.e., Zn2+ ions) by loss of electrons. Hence, Zn has been oxidised. CuSO4 (i.e., Cu2+) has changed into Cu by gain of electrons. Hence, CuSO4 has been reduced. Thus, the above reaction is a displacement reaction as well as a redox reaction.
(ii)

Also check: CBSE Class 10 Science Sample Paper 2022-23
#CBSE #Class #Chemistry #Chapter #Important #Questions #Answers
