Jagran Josh
Revision Notes for CBSE Class 10 Science Chapter 4: CBSE Class 10 students can now revise their Science syllabus in a few minutes with the help of the quick revision notes or short notes presented by Jagran Josh. In this article, you will be getting the revision notes for CBSE Class 10 Science Chapter – Carbon and its Compounds. These notes have been prepared according to the revised syllabus that has been released for the current academic session. Our subject experts have taken care of including all important concepts to help you revise the chapter effectively and prepare for the class tests and annual board examinations in the best possible way. You can also solve the questions given at the end of the notes for self-assessment.
Also Read|
CBSE Class 10 Science Deleted Syllabus 2023-24
CBSE Class 10 Science Sample Paper 2023-24
Revision Notes for CBSE Class 10 Science Notes for Chapter 4 Carbon and its Compounds:
Covalent Bond: The bond formed by mutual sharing of electron pairs between two atoms in a molecule is known as Covalent Bond.
Covalent bonding in carbon compounds
The atomic number of carbon is 6 and its electronic configuration is 2, 4. Therefore, needs to gain or lose four electrons to attain noble gas configuration. But it is not possible for carbon to form ionic bonds due to the following reasons:
(i) It could gain four electrons forming C4- cation. But it would be difficult for the nucleus with six protons to hold on to ten electrons.
(ii) It could lose four electrons forming C4+ cations. But it requires a large amount of energy to remove four electrons.
Carbon overcomes this problem by sharing its valence electrons with other carbon atoms or with atoms of other elements. Thus, carbon forms covalent bonds.
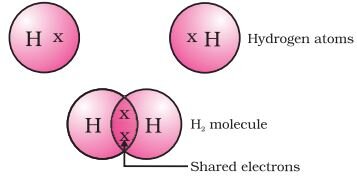
Formation of hydrogen molecule (Single Bond):
The atomic number of hydrogen is 1. Hence it has one electron in its K shell and it requires one more electron to fill the K shell. So two hydrogen atoms share their electrons to form a molecule of hydrogen, H2.
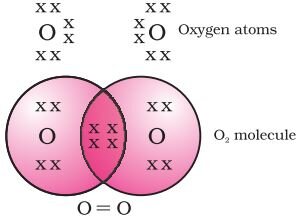
Formation of oxygen molecule (Double Bond):
The atomic number of oxygen is 8. So it has six electrons in its L shell and it requires two more electrons to complete its octet. So each atom of oxygen shares two electrons with another atom of oxygen. The two electrons contributed by each oxygen atom give rise to a double bond between the two atoms.
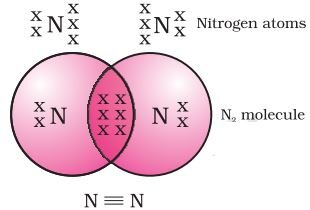
Formation of nitrogen molecule (Triple Bond)
Nitrogen has the atomic number 7. So it has five electrons in its L shell and it requires three more electrons to complete its octet. So, in order to attain an octet, each nitrogen atom in a molecule of nitrogen contributes three electrons giving rise to three shared pairs of electrons that results in a triple bond between the two atoms.
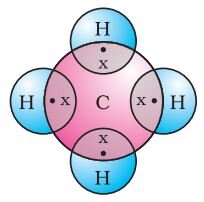
Formation of methane (CH4)
Also Check: CBSE Class 10 Science Best Study Plan for Board Exam 2021
Allotropes of Carbon
Different forms of an element that have same chemical properties but different physical properties are known as Allotropes.
There are three allotropes of carbon – Diamond, graphite and Fullerene
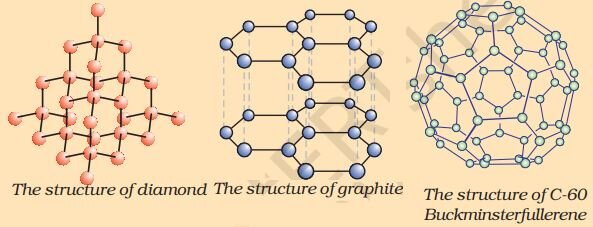
(i) Diamond
In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure.
→ It is the hardest substance known.
→ It has high melting point.
→ It is a bad conductor of electricity.
→ It is used in making jewellery. It is also used in cutting and drilling tools.
(ii) Graphite
→ Each carbon atom is bonded with other three carbon atoms in the same plane forming hexagonal rings.
→ These rings are stacked over each other through weak Van der Waals forces.
→ Graphite is also a very good conductor of electricity.
→ It is used as dry lubricant.
→ It is used in making pencil lead.
(iii) Fullerene
→ C60, also known as Buckminsterfullerene, is the most common form of fullerenes.
→ It has carbon atoms arranged in the shape of a football.
→ It consists of 60 carbon atoms arranged in 12 pentagons and 20 hexagons,
Versatile nature of carbon
Carbon is included in ten formation of a variety of compounds. This is due to the following two reasons:
(i) Catenation: Carbon has the unique ability to form covalent bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation.
Carbon exhibits catenation property due to the following reasons:
→ Size of the carbon atom is very small. This enables its nucleus to hold the shared pair of electrons strongly.
→ The carbon-carbon bond is quite strong.
(ii) Tetravalent Nature: Since carbon has a valency of four, it is capable of bonding with four other atoms of carbon or atoms of some other mono-valent element.
Hydrocarbons
Compounds of carbon and hydrogen are known as hydrocarbons.
For example – Methane (CH4), Ethane (C2H6), etc.
Saturated hydrocarbons: These hydrocarbons have all carbon-carbon single bonds. These are known as alkanes.
General formula = CnH2n+2
where n = number of carbon atoms
Unsaturated hydrocarbons: These hydrocarbons have at least one carbon-carbon double or triple bond.
→ Hydrocarbons with carbon-carbon double bond are known as alkenes. General formula = CnH2n
→ Hydrocarbons with carbon-carbon triple bond are known as alkynes. General formula = CnH2n-2
Structural Isomers
Isomers
The compounds with the same molecular formula and different physical or chemical properties are known as isomers.
Compounds with identical molecular formula but different structures are called structural isomers.
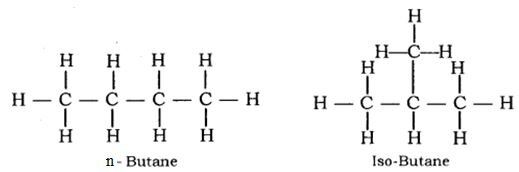
Isomers of butane:
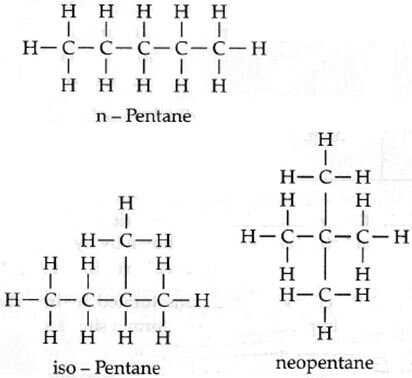
Isomers of pentane:
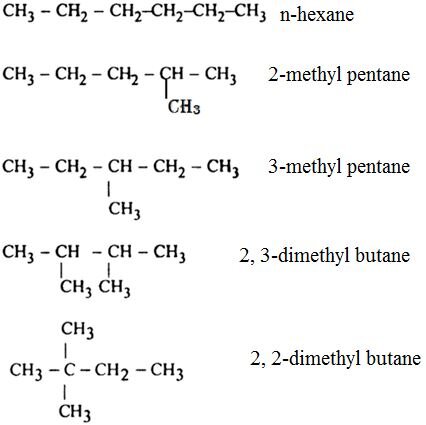
Isomers of hexane:
Homologous series
A series of organic compounds having the same functional group and chemical properties and in which the successive members differ by CH2 unit or 14 mass units is known as Homologous series.
Characteristics of homologous series are:
- The successive members in homologous series differ by CH2 unit or 14 mass unit.
- All compounds of a homologous series have the same functional group.
- All compounds of homologous series shows similar chemical properties due to the addition of same kind of functional group throughout the chain.
For example,
Homologous series of alkanes is CH4 (Methane), C2H6 (Ethane), C3H8 (Propane),….
Nomenclature of Carbon Compounds:
1. Identify the number of carbon atoms in the compound.
2. Functional group is indicated either by prefix or suffix.
|
Functional Group |
Suffix |
|
Alkene |
ene |
|
Alkyne |
yne |
|
Alcohol |
ol |
|
Aldehyde |
al |
|
Ketone |
one |
|
Carboxylic acid |
oic acid |
3. If a suffix is added, then final ‘e’ is removed from the name eg. methanol (methane – e = methan + ol).
Questions for self-assessment:
Q. Draw the electron dot structure of ethylene.
Q. How are covalent compounds different from ionic compounds?
Q. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Q. State any two characteristics of homologous series.
Q. Write first four members of homologous series of alkenes.
#CBSE #Class #Science #Short #Notes #Chapter #Carbon #Compounds #Based #Revised #Syllabus
