Jagran Josh
Thermal Properties of Matter Formulas & Definitions: Students can find a list of important diagrams and formulas for CBSE Class 11 Physics Chapter 10, Thermal Properties of Matter. Use the PDF download link attached below to save the formula sheet.
CBSE Class 11 Physics Thermal Properties of Matter Formulas: Find here a list of important formulas, definitions, graphs, and diagrams for CBSE Class 11 Physics Chapter 10, Thermal Properties of Matter. Also, find attached a PDF download link for the same. This formula sheet will assist you in your preparation for the upcoming annual examination in 2024. Also find attached links for other various important resources required for the preparation of examinations, here.
Thermal Properties of Matter is an interesting chapter from the Class 11 Physics NCERT textbook since it consists of amusing phenomena related to matter and its heat and temperature concepts. The chapter consists of some important formulas and graphs from the exam’s point of view. Various numerical problems can be solved by using these formulas and derivations presented in this chapter. Students can use this formula sheet when solving those numericals since they don’t have to keep searching for formulas scattered all across the chapter.
Formula Sheet or Formula Page is an important piece of paper with an integrated list of formulas, definitions., graphs, and diagrams. These are really important from the examination’s point of view and are handy. Students can refer to these whenever required especially while practicing sums for the exam. Formula pages are great revision- partner buddies. Consistently going through the formulas present on the sheet can help you easily memorize them and use them as per their application.
Class 11 Physics Thermal Properties of Matter Formula Sheet
Formulas:
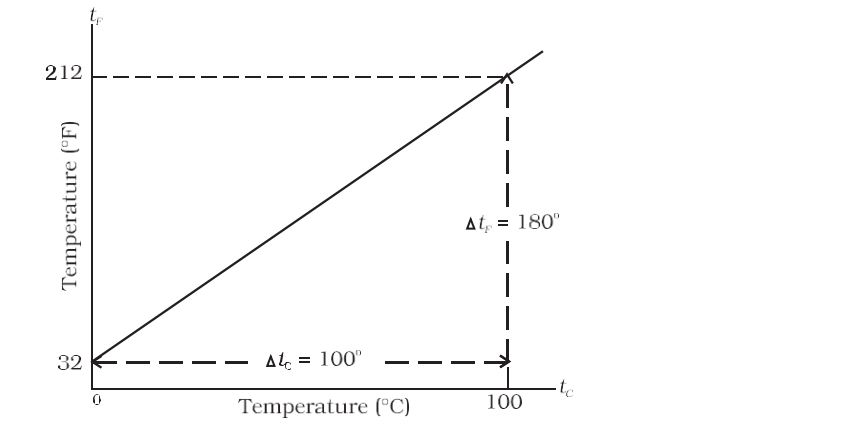
Relationship between two scales, Celsius and Fahrenheit –
Ideal Gas Equation–
Temperature at Kelvin Scale-
Linear Expansion-
Area Expansion-
Volume Expansion-
Coefficient of Linear Expansion-
Coefficient of Volume Expansion–
coefficient of volume expansion for ideal gas-
Relationship between linear expansion and volume expansion-
Heat Capacity-
Specific Heat Capacity-
Molar Specific Heat Capacity-
Latent Heat-
Thermal Conductivity- The constant of proportionality K is called the thermal conductivity of the material.
Wien’s Displacement Law-
Stefan Boltzmann Law-
Emissivity-
Newton’s Law of Cooling-
Definitions:
Heat– It is the form of energy transferred between two (or more) systems or a system and its surroundings by virtue of temperature difference. The SI unit of heat energy transferred is expressed in joule (J).
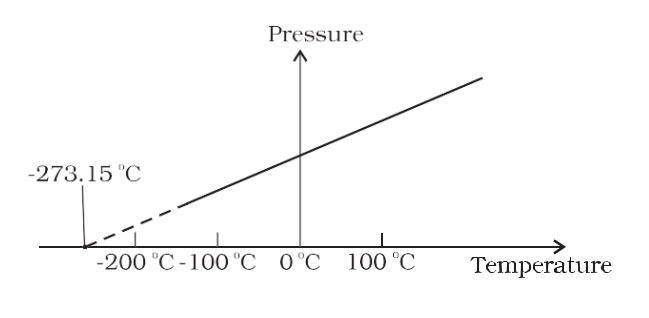
Charles Law– When the pressure is held constant, the volume of a quantity of the gas is related to the temperature as V/T = constant. This relationship is known as Charles’ law.
Thermal Expansion– The increase in the dimensions of a body due to the increase in its temperature is called thermal expansion.
Linear Expansion- The expansion in length is called linear expansion.
Area Expansion- The expansion in area is called area expansion.
Volume Expansion- The expansion in volume is called volume expansion.
Heat Capacity- The change in temperature of a substance, when a given quantity of heat is absorbed or rejected by it, is characterized by a quantity called the heat capacity of that substance. We define heat capacity as S.
Specific Heat Capacity– Every substance has a unique value for the amount of heat absorbed or given off to change the temperature of its unit mass it by one unit. This quantity is referred to as the specific heat capacity of the substance.
Molar Specific Heat Capacity– If the amount of substance is specified in terms of moles m, instead of mass m in kg, we can define heat capacity per mole of the substance by heat capacity divided by mole, where C is called molar specific heat capacity.
Molar specific heat capacity at constant pressure– If the gas is held under constant pressure during the heat transfer, then it is called the molar specific heat capacity at constant pressure and is denoted by Cp.
Molar specific heat capacity at constant volume– If the volume of the gas is maintained during the heat transfer, then the corresponding molar specific heat capacity is called molar specific heat capacity at constant volume and is denoted by Cv.
Calorimeter– A device in which heat measurement can be done is called a calorimeter.
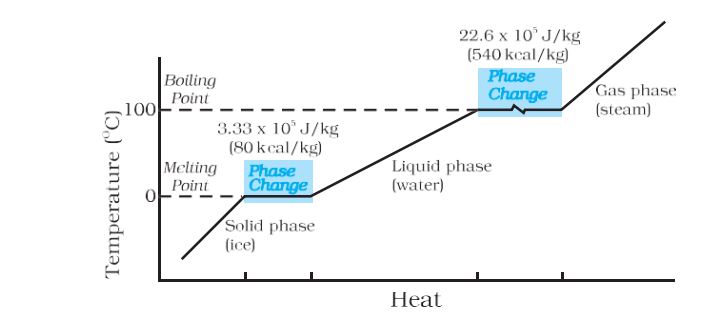
Melting– The change of state from solid to liquid is called melting.
Freezing- fusion and from liquid to solid is called freezing.
Melting Point- The temperature at which the solid and the liquid states of the substance are in thermal equilibrium with each other is called its melting point.
Normal Melting Point– The melting point of a substance at standard atmospheric pressure is called its normal melting point.
Vaporisation– The change of state from liquid to vapor (or gas) is called vaporization.
Boiling Point- The temperature at which the liquid and the vapor states of the substance coexist is called its boiling point.
Normal boiling point– The boiling point of a substance at standard atmospheric pressure is called its normal boiling point.
Sublimation– The change from solid state to a vapor state without passing through the liquid state is called sublimation, and the substance is said to sublime.
Latent heat of fusion– The latent heat for a solid-liquid state change is called the latent heat of fusion (Lf)
Latent heat of vaporisation– The latent heat for a liquid-gas state change is called the latent heat of vaporisation (Lv).
Conduction– Conduction is the mechanism of transfer of heat between two adjacent parts of a body because of their temperature difference.
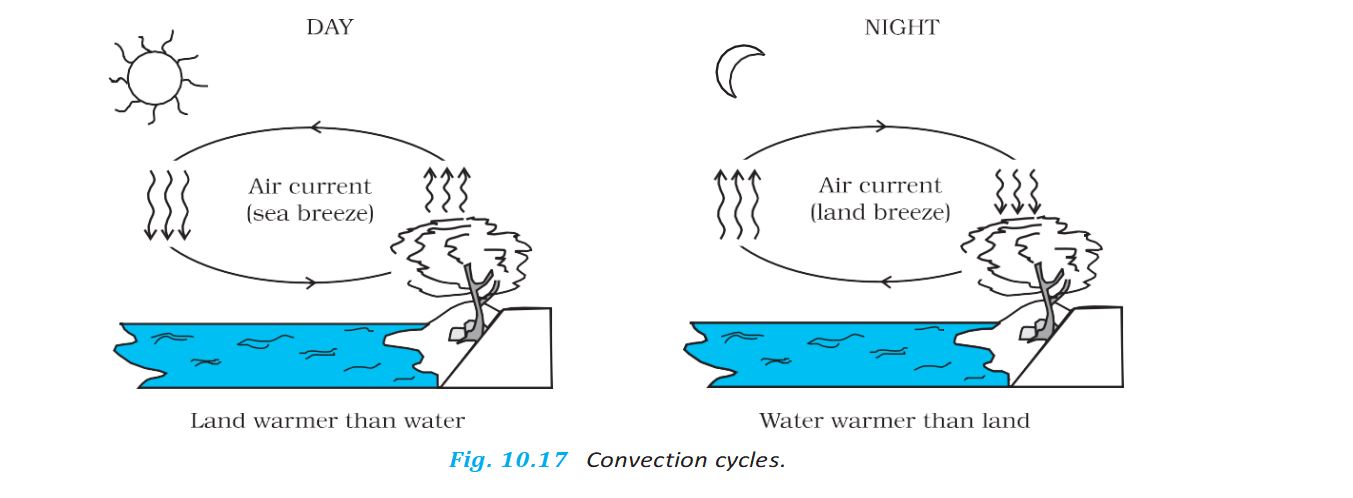
Convection- Convection is a mode of heat transfer by actual motion of matter. It is possible only in fluids.
Graphs:
- Plot of Celsius vs. Fahrenheit-
- Pressure vs Temperature-
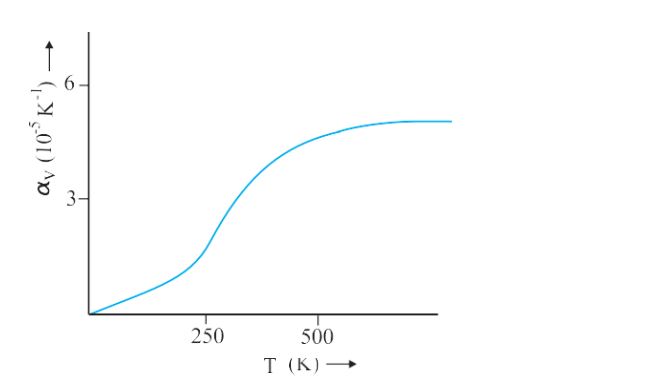
- Coefficient of Volume as a function of temperature-
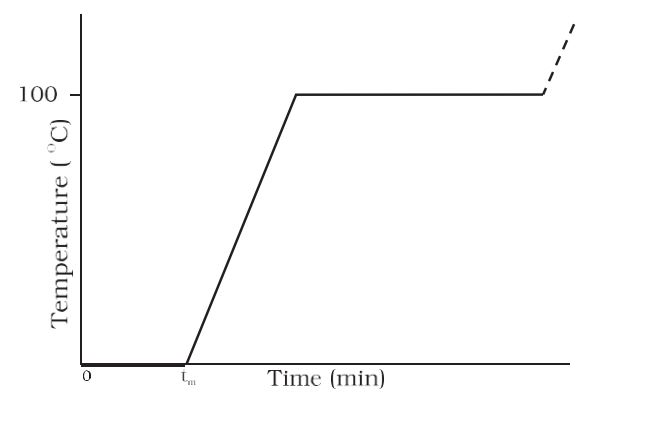
- Temperature vs. Time showing the changes in the state of ice on heating-
- Temperature versus heat for water at 1 atm pressure–
Diagrams:
Convection Cycles-
To download the complete Formula Sheet for CBSE Class 11 Physics Chapter 10 Thermal Properties of Matter, click on the PDF download link below
Also Find:
Formula Page for CBSE Class 11 Physics Chapter Mechanical Properties of Solids
Formula Page for CBSE Class 11 Physics Chapter Mechanical Properties of Fluids
CBSE Class 11 Physics Syllabus 2023-2024
CBSE Class 11 Physics Deleted Syllabus 2023-2024
#CBSE #Class #Thermal #Properties #Matter #Formula #List #Definitions #Graphs

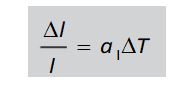
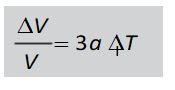
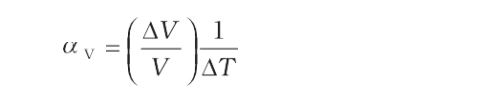

.JPG)