Jagran Josh
Gay-Lussac’s Law Formula: Check Gay-Lussac’s Law application, formula, and notes here.
Gay-Lussac’s law of gaseous volume: Class 11 and 12 Chemistry students must have heard of the famous concept named Gay-Lussac law. This law is also known as the pressure-temperature relationship, which is a fundamental concept in thermodynamics. This law was named after the French chemist Joseph Louis Gay-Lussac, who formulated it in 1808. Understanding this law helps provide crucial insights into how the pressure and temperature of a gas are interrelated. In this article, you will delve into the details of the Gay-Lussac law, its significance, and its practical applications.
What is Gay-Lussac’s Law?
According to Gay-Lussac’s Law of combining volumes, the pressure exerted by the gas (of a given mass and kept at a constant volume) is directly proportional to the absolute pressure of the gas at a fixed mass and the volume.
P ∝ T; P/T = k
Where:
- P is the pressure exerted by the gas.
- T is the absolute temperature of the gas.
- K is a constant.
Relationship Between Pressure and Temperature
At constant volume and mass, the relationship between pressure and temperature will look like this:

The graph depicts a direct relationship between pressure and temperature. This shows that the pressure of gas is constantly reduced with a decrease in temperature.
Gay-Lussac’s Law and Derivation
Gay-Lussac’s Law of Gaseous Volume states that the ratio of initial gas pressure and temperature is equal to the ratio of final pressure and temperature, as both are directly proportional (at constant gas mass and volume). This formula can be expressed as follows:
(P1/T1) = (P2/T2)
Where:
- P1= the initial pressure.
- T1= the initial temperature.
- P2= the final pressure.
- T2= the final temperature.
P1/T1 = k
P2/T2 = k
Therefore, P1/T1 = P2/T2 = k.
Or, P1T2 = P2T1
Examples of Gay-Lussac’s Law
- One of the most famous applications of Gay-Lussac’s Law is in hot air balloons. As the air inside the balloon is heated, its temperature and pressure increase, causing the balloon to rise. This demonstrates the law in action and provides a breathtaking experience for passengers.
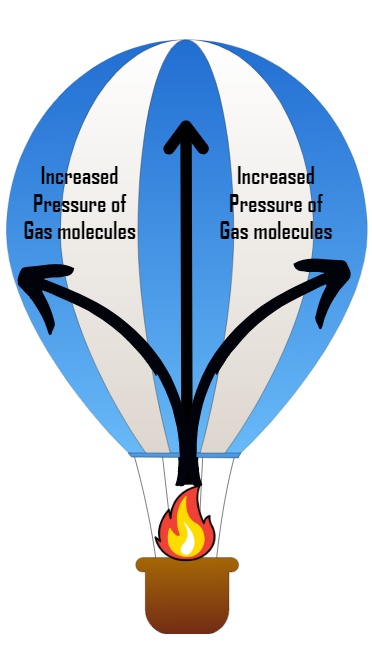
- The packaging of pressurised aerosol cans is done based on this method. The gases are filled in cans at room temperature to bypass the high pressure of gases on the can walls. This is why you will see the labels stating to keep the cans in a cool environment to avoid explosions.
Solved Gay-Lussac’s Law Question Examples
Q1. At a temperature of 300 K, the pressure of the gas in a deodorant can is 3 atm. Calculate the pressure of the gas when it is heated to 900 K.
Sol.
Initial pressure, P1 = 3 atm
Initial temperature, T1 = 300K
Final temperature, T2 = 900 K
Therefore, final pressure (P2) = (P1T2)/T1 = (3 atm X 900K)/300K = 9 atm.
Q2. The pressure of a gas in a cylinder when it is heated to a temperature of 250K is 1.5 atm. What was the initial temperature of the gas if its initial pressure was 1 atm?
Sol.
Given,
Initial pressure, P1 = 1 atm
Final pressure, P2 = 1.5 atm
Final temperature, T2 = 250 K
As per Gay-Lussac’s Law, P1T2 = P2T1.
Therefore, T1 = (P1T2)/P2 = (1 atm X 250K)/(1.5atm) = 166.66 Kelvin.
#GayLussacs #Law #Formula #Definition #Statement #Questions
