Jagran Josh
Law of Mass Action Formula: Check the Law of Mass Action application, formula and notes here.
Application Of Law of Mass Action: The law of mass action is one of the major topics taught to Class 11 chemistry students. This topic comes under the Equilibrium chapter of the NCERT Class 11 Chemistry textbook. In the final exams of classes 11 and 12, many questions can be formed from the law of mass action. The law of mass action formula, the law of mass action example,
The law of mass action derivation can be called The Law of Mass Action, formulated by Cato Maximilian Guldberg and Peter Waage in the 19th century Thus, we can say the law of mass action was given by these two. To know more about the Law of Mass Action, read this article.
Read: Henry’s Law
What is the Law of Mass Action?
The law of mass action states that the rate of a chemical reaction is directly proportional to the product of the concentrations of the reactants, each raised to the power of their respective coefficients in the balanced chemical equation.
According to the law of mass action, at chemical equilibrium the ratio of the reactants and product concentrations is constant.
The Equilibrium Constant (Kc)
Mathematically, the Law of Mass Action can be expressed as follows:
aA+bB⇌cC+dD
Where:
- a and b are the stoichiometric coefficients of the reactants A and B, respectively.
- c and d are the stoichiometric coefficients of the products C and D, respectively.
- The double arrow (⇌) indicates that the reaction can proceed in both the forward and reverse directions.
According to the Law of Mass Action, the rate of the forward reaction (rf) is directly proportional to the product of the concentrations of the reactants raised to their respective coefficients:
rf ∝ [A]a[B]b
Similarly, the rate of the reverse reaction (rr) is directly proportional to the product of the concentrations of the products raised to their respective coefficients:
rr ∝ [C]c[D]d
At equilibrium, the rates of the forward and reverse reactions become equal, leading to the equilibrium expression:
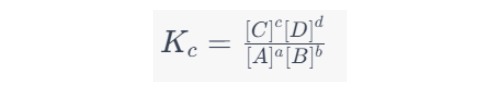
Where Kc is the equilibrium constant, which is unique for each chemical reaction at a given temperature.
|
Equilibrium Constant Representation |
Expressed in terms of |
Expression |
|
Kc |
Concentrations of reactants and products |
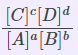 |
|
Kp |
Partial pressures of reactants and products. (only for the substances which are in a gaseous state) |
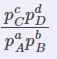 |
|
Kx |
Mole fractions of reactants and products |
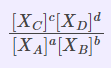 |
Kp=Kc(RT)Δng
Kp= Kx.PΔng
Δng= (moles of gaseous products)-(moles of gaseous reactants)
Law of Mass Action Formula
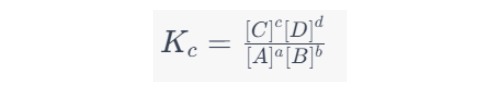
Significance of the Law of Mass Action
- Predict Equilibrium: The Law of Mass Action allows chemists to predict the direction in which a reaction will proceed to reach equilibrium.
- Quantitative Analysis: The Law of Mass Action provides a foundation for quantitative analysis in chemistry. By knowing the initial concentrations of reactants and products, as well as the equilibrium constant, scientists can calculate the concentrations of substances at equilibrium.
- Industry Applications: The Law of Mass Action is vital for optimizing reaction conditions and yields.
- Le Chatelier’s Principle: The law is closely related to Le Chatelier’s Principle, which states that when a system at equilibrium is subjected to a change in temperature, pressure, or concentration, it will shift its position to counteract that change.
Solved Law of Mass Action Examples
1. Calculate Kc for 2NO2⇌N2O4. Consider [N2O4] = 0.0417 mol L-1and [NO2] = 0.0165 mol L-1.
Solution
The Equilibrium constant for this reaction can be calculated using the Law of Mass Action formula:
[N2O4][NO2]2=Kc
Using [N2O4] = 0.0417 mol L-1 and [NO2] = 0.0165 mol L-1 as starting points,
153=Kc=0.04170.01652
2. The following concentrations were obtained for the formation of NH3from N2and H2 at equilibrium at 500K. [N2] = 1.5 × 10–2M. [H2] = 3.0 ×10–2 M and [NH3] = 1.2 ×10–2M. Calculate equilibrium constant.
Solution
The equilibrium constant for the reaction, N2(g) + 3H2(g) ⇌ 2NH3(g) can be written as,
Kc = [NH3(g)]2/[N2(g)][H2(g)]3 = (1.2×10-2)2/(1.5×10-2)(3.0×10-2)3 = 0.106×104 = 1.06×103
Also Read:
#Law #Mass #Action #Chemical #Equilibrium #Formula #Definition #Examples #State #Explain
